
Research Theme
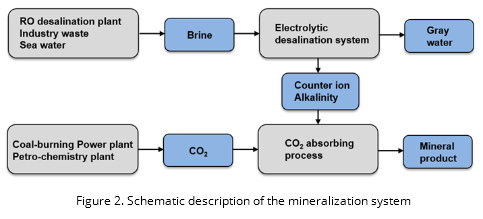
Through the overall mineralization system, the hydrolysis of CO2 is the first step of the rate-limiting reaction during the formation of mineralization so that the absorbents that are initially contained in the system selectively capture only CO2. Herein, the biotic (e.g., carbonic anhydrase (CA) and bio-inspired adhesives) as well as abiotic absorbent (e.g., alkali and/or amine based chemicals) are investigated in this research. For the next step, the carbonate (CO32-) and bicarbonate (HCO3-) ions cause precipitation when only the positive counter ions (e.g., Na+, K+, Mg2+, and Ca2+) are transferred by the electrical attraction, herein, any salt-containing solution, including such as industrial waste, sea water, even brine with low concentration, can serve as a counter-ion supplier. The minerals recovered from this electrolytic system contain both the carbonate and bicarbonate ions; however, the components change their form mutually according to the simple treatment of pH and temperature as necessary. Consequently, our approach can achieve elimination of CO2 from the flue gas (typically 10~15%) and at the same time produce valuable products for industrial applications. Also, the pure gas produced by the reaction of oxidation/reduction on the electrode can also be recovered, and these include chlorine (Cl2) gas, and hydrogen gas (O2) on the anodic part, and hydrogen (H+) gas on the cathodic part, which is another economic benefits.
Along with the development of electrolytic system, the systems will be optimized through simulation considering ever-changing economic variables (e.g., cost of raw material, transport fee, exchange rate, and taxes) that are closely related with this system; in addition, the adaption of experimental results into a systemic approach is necessary. The electrolytic system is a key process in terms of maintaining alkalinity for CO2 hydrolysis and supplying sufficient counter ions; nevertheless, the system has a bottle-neck due to the inevitable cost incurred by the supplement of electric energy, and sensitive effects caused by scale-up. Therefore, optimization by simulating this electrolytic system is necessary for reducing the operating cost as well as investments, furthermore, the capacity of CO2 in mineralization according with the operation time also can be predicted.
In this project, the simulation will be carried out in two steps: 1) optimization procedure for the mineralization system, and 2) real-time optimization (Figure 2, Figure 3).
1) Mineralization process modeling
1-1) CO2 absorption process modeling
1-2) Electrolytic system modeling for supplementation of counter-ions and alkalinity production
1-3) Model integration and validation
2) Real-time optimization
2-1) Sensitivity analysis to select and manipulate variables (Monte-Carlo simulation)
2-2) Constructing objective function for optimization (Multiple scenarios)
2-3) Model predictive control in consider of optimized set point

- Integrative processes of CO2 mineralization and brine demineralization
- CO2 capturewith abiotic/chemical catalysts with improved performance
- Multi-functional CA-hybrid platforms based on the protein display on genetically modified bacteria Optimization and cost analysis of overall process
- Integrated CO2 absorption and conversion system based on an electrolytic desalination system.
- Enhancement of water electrolysis for effective and continuous CO2 capture with enlarged capacity.
- Applicable and stable system for any absorbents in large range of operating temperature and electrical energy.
- Surface display of carbonic anhydrase (CA) using C. glutamicum in terms of a biological approach.
- Process optimization with simulation.


